How about I tell you, that the first person to live up to 150 years has already been born?
It’s not a blind fact, in fact, humans (we) were able to stretch our lifespan from a ripe old age of 41 years in the 1800s to an astounding global average of 72.6 years[1].
But adding a few more years on the global average isn’t enough, like everything else we ‘want’ more of it, a long and perhaps in the future immortal life.
We still can stretch our living years but no one wants to live in the vain of old age, surrounded by disease, pain, and frailty.
Or consider being like “Hydra”, a biologically immortal organism, it keeps reproducing (by budding of course), on and on and on, there is no such evidence that it actually ages or such.
A species of jellyfish “Turritopsis dohrnii”, is something of a wonderful thing, it leaps in back in the development process in response to stressful conditions, making it immortal.
Only if our systems were simple enough but it’s not like that, is it?
So is there a way to make us biologically immortal beings?
What is Aging?
Aging refers to physiological and cellular changes we experience during our lifetime.
Aging simply exists because we’ve “evolved” to age but evolving for something doesn’t always imply it is for the good of the species.
We age because our systems aren’t robust enough to tackle reproduction, DNA repair, and survival all at once.
The existing old cells give rise to new ones, the more a cell divides the more aging factors accumulate in a cell.
Biological Aging
As it may sound, biological aging is quite different from chronological aging; chronological age is the years that have passed since one was born (including those days when you’ve felt dead inside).
One can have a chronological age of 20 years but their biological age could differ based on their lifestyle.
Biological age can’t be measured easily by normal clocks, different cells, and organisms age in different ways.
Biological aging is embarked by cellular damage that accumulates in cells over time and it is determined by both environmental and genetic factors.
There are many factors taken into consideration while determining biological age:
- Lifestyle
- Nutrition
- Diseases and other health conditions
- Smoking habits
One’s biological age could be greater or lesser than their chronological age.
Phases of Biological Aging
The first phase of aging is the “early age” or juvenile phase; changes or growth that occur don’t result in any kind of impairment or damage to the intra-cellular structure of the body.
The second phase of aging is the “intermediate age” or reproductive phase; this phase marks the onset of the accumulation of aging factors in the cells.
Now the third and final phase of aging, the “late age” or senescent phase, after all the aging factors accumulated in the intermediate phase, this phase coincides with the death of an organism, thus ending the lifespan.
All these phases happen when an organism dies by the means of nature, i.e, a natural death.
Natural Death
The natural death of an organism is considered to be natural if there’s no external influence on the death.
Natural death occurs when the body shuts down due to any disease such as cancer or heart disease.
Biological Clocks – How do we Biologically Measure Aging?
The epigenetic clock is a relevant way of determining the biological age of a person.
Epigenetic clocks use data to calculate biological age on the basis of hundreds of sites across the genome of an individual, bound to methyl groups – a type of epigenetic modification.
Epigenetic Clock – Horvath clock
The epigenetic clock is also known as the Horvath clock, a name derived from the inventor of the clock, Steve Horvath.
In the year 2011, Steve and colleagues developed a method of estimating biological age by saliva samples and studying DNA modifications such as DNA methylation (DNAm).
Epigenetic clocks are vastly used to measure the age of cells and tissues in the organism.
Epigenetic clocks are biomarkers of aging, based on the methylation of DNA, another form of Epigenetic modification.
GrimAge
In the year 2019, a team led by Ake T. Lu of UCLA with Horvath developed a new biological clock called DNAm GrimAge.
This clock takes the person’s gender, smoking history, chronological age, and blood mortality markers into account while predicting the age[2].
A higher score in this predicting module means a greater mortality/mobility risk.
DNAm GrimAge is a reliable predictor of mortality independent of genetic influences.
Why are Epigenetic clocks Important?
Suppose scientists are trying to develop an age-related medication, to slow down or reverse the aging cycle.
To test if it actually works, they need a test subject to test whether the medication works on human beings and to see the long-term effect.
It’s quite difficult because one will have to observe the entire lifespan of that particular human being, to see if the medication actually worked to improve the lifespan.
We can test many age-related interventions by using the Epigenetic clock without consuming a hefty amount of time.
If we somehow are able to understand the working of these kinds of clocks it could lead us to the root cause of aging.
Why do we age? – No Definite Cause
The quest to find the root cause of aging is going on for pretty much forever.
To be able to find the root cause of aging is surely gonna help scientists create a reliable anti-aging drug or some kind of intervention, that’ll help us to tackle aging in an efficient way.
Either Live Long or Have Sex – Disposable Soma Theory
Given by Thomas Kirkwood (Newcastle University)
It states that an organism has a limited amount of resources, either for growth and reproduction or DNA maintenance.
Meaning that an organism can breed fast, but if they do so, that would result in a reduced investment of resources in DNA maintenance (really need to sort out priorities here).
Individuals don’t live forever because nature doesn’t select to do both, longevity and reproduction, not in the world where the body works perfectly fine to pass down the body’s genes.
So, either an organism can breed fast and die young or breed slow and maintain a robust, healthy “soma” (body).
This theory is primarily inclined toward the methods of cellular aging.
The theory suggests that increased investment in cellular repair would result in an “individual’s” fitness but not of the species.
One of the reasons this theory isn’t considered relevant is that it does not postulate any mechanism which shifts energy to cellular repair over reproduction.
It is sexually biased as well because most of the reproduction load is on the female counter-part and this theory still can’t explain why females live longer than most men, (at this point, you can conclude that having or not having sex got nothing to do with aging :p).
DNA Machinery is Messed Up – Error Catastrophe Theory
Given by Leslie Orgel
It postulates errors in the DNA copying process lead to mutation (errors) in genes, including those cellular molecules that are essential for the protein-making machinery of DNA.
This process completely disrupts DNA copying mechanisms and error is repeated again and again, till a person’s genome is copied to oblivion, leading to aging and eventually death.
A Mathematical Take on Aging – Information Theory of Aging
This theory is based on mathematician Claude Shannons’s work on information theory.
David Sinclair describes it as aging is caused by the accumulation of “noise” in DNA sequences and this noise degrades replicating efficiency of DNA over time.
“Noise” here suggests the variation in genetically similar cells even within the same tissue[3].
This noise in cells results in varied gene expression levels, variation in gene expression infers that the cell with noise will have a different appearance, response to external stimuli, ability to process information, et cetera.
This theory in particular suggests that this noise accumulation in cells leads to aging.
Hallmarks (Symptoms) of Aging
Now, there are certain “hallmarks” or say indicators of the fact that we’re aging on a cellular level.
These hallmarks are accurate indicators of aging and its diverse symptoms.
Genomic instability caused by mutations in DNA
The genome is the complete set of genetic information in an organism, the genome is stored in long strands of DNA, called chromosomes.
For example, the human genome consists of 23 pairs of chromosomes including one pair of sex chromosomes.
DNA forms genes, gene forms chromatin, chromatin forms chromosomes, and chromosomes form genome.
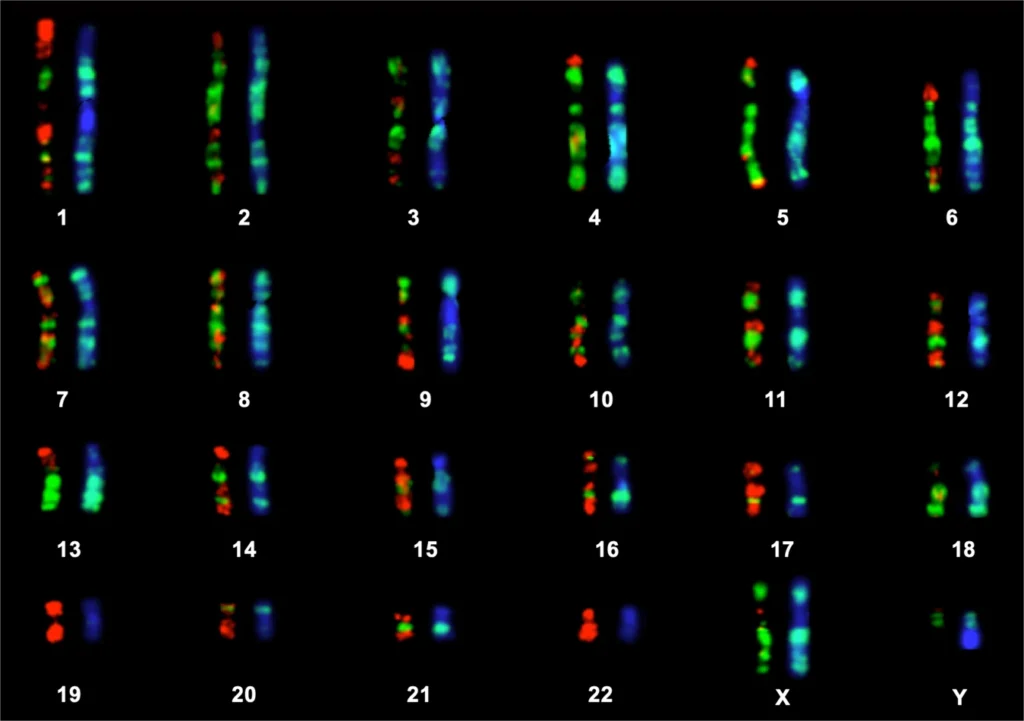
Genomic instability is an outcome of mutations in DNA repair genes.
Genomic instability refers to DNA alterations, varying from point mutations (insertion, deletion, or shifting of a single nucleotide in DNA), to insertion and deletion of genes to whole chromosomal rearrangements, which irreversibly change the whole genome.
These mutations in DNA accumulate in aging tissues and result in the progressive decline of organ functionality.
The preservation of genomic stability is important for cellular integrity to prevent flaws in DNA replication[4].
Genomic instability is also an aspect of the cause of cancer, in hereditary cancers[5].
Genomic instability results in a shorter cell cycle, which means early cell death and accumulation of senescent cells, bypassing immunological and intracellular control systems, which gives rise to malignant and cancerous cells.
Telomere Attrition
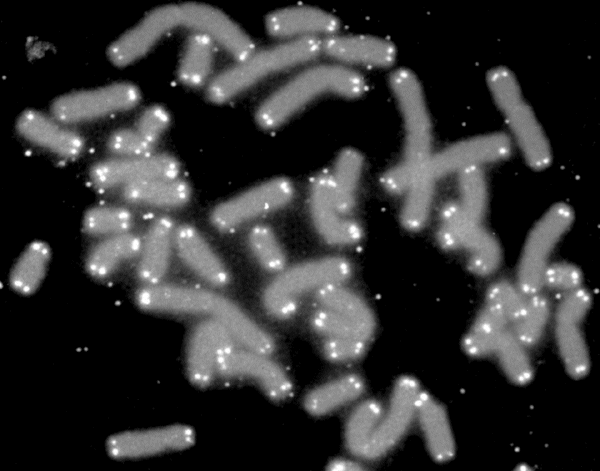
Telomere attrition (shortening) is the continuous loss of telomeres (or protective caps) present on our chromosomes.
This attrition limits the number of times our cells can divide, slowly diminishing the number of cells in our vital organs, which of course, leads to senescence and eventually death of the cell[6].
After a cell reaches senescence it is disposed of by the immune system, by naturally programmed cell death, it’s called “apoptosis”.
Telomere attrition also results in increased odds of lifestyle-related diseases, thus shortening the life span.
One can take hold of the rate of telomeres decreasing or increasing by certain lifestyle changes.
Alterations to the Epigenome
The epigenome, if I were to explain what is epigenome in simpler words, it’d be like this,
If the genome is a piano, then the epigenome would be a pianist.
The epigenome is stored in a complex structure called chromatin, just like genetic information is stored in DNA.
Epigenome directs a cell, and how it behaves, it’s one neuron different from a skin cell.
Without epigenetic information, cells lose their identity, we lose functionality, hence complete chaos.
As the epigenome determines which genes are turned on and off, it also regulates the production of proteins in cells.
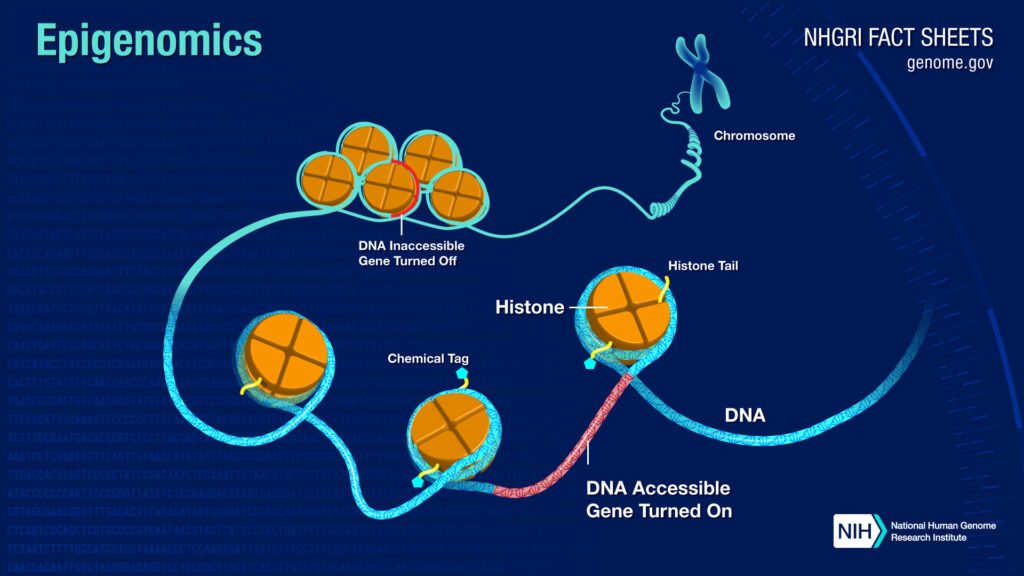
Epigenome modifications vary among an individual, even within the same cell in a tissue.
A common modification to the epigenome is called DNA methylation, as is evident by the benevolence of methyl (a molecule consisting of carbon and three hydrogen atoms).
In the methylation of DNA little methyl groups attach to it, when this happens that specific gene is turned off or silenced and it doesn’t manufacture any protein[7].
Another frequent epigenetic change is histone modification, histones can be modified by the addition of methyl or acetyl groups, which affects the intensity of how tightly is DNA wrapped around a histone, which further results in whether a gene can be turned off or not[7].
Errors in the epigenetic process, modification of a wrong gene, or misprision to add a certain chemical group to a particular gene or histone, result in abnormal gene activity which furthermore results in degenerative disorders.
Degenerative disorders like osteoarthritis, osteoporosis, Alzheimer’s, etc.
Alzheimer’s is a progressive disease, which annihilates the memory and other important functions of the brain.
Brain cells and synapses degenerate and die eventually, completely destroying memory forming ability of the brain.
Alzheimer’s is caused by a bunch of factors which include age-related factors affecting the brain, along with genetic and lifestyle factors.
Accumulation of Senescent Cells
Senescent cells, or what I like to call zombie cells, are hindered from growth and kind of just lying there and spreading toxicity.
Instead of just dying by “apoptosis”(naturally programmed cell death), these cells lose the ability to replicate and just accumulate inside tissues/ organs.
These zombie cells inhibit tissue regeneration by producing some compounds like SASP (senescence-associated secretory phenotype).
SASP plays a major role in wound healing but over-accumulation of senescent cells declines organ functionality.
Declining organ functionality by the virtue of zombie cells is a significant cause and indicator of aging.
There are other hallmarks related to aging as well but stated above are the important ones.
Just for the sake of context, here are other “Hallmarks Related To Aging”:
- Proteostasis
- Deregulated nutrient sensing caused by metabolic changes
- Dysfunction of mitochondria
- Stem cell exhaustion
- Altered intercellular communication
Gerontologists and geneticists agree on the fact that one can address these symptoms of aging to slow down the aging process which can help us hinder forestall aging-related disorders.
Vitality (Longevity) Genes – Aging Prevention
Scientists have found a family of genes, which are responsible for an organism’s ability to resist extreme conditions and scarcity of food & water as such.
These genes provide organisms the ability to keep their natural defense and repair activities going, in a stressful environment.
By enhancing bodily functions for survival, these genes escalate the possibility of an individual’s survival in a crisis.
And if these genes are activated long enough in the body, they drastically improve the health and lifespan of an individual.
The prominent ones we’re gonna be discussing here are “Sirtuins”, named after SIR2 genes found in yeast (SIR1 yet to be discovered).
Sirtuins (SIRT3) – Boosts Longevity in Humans
Sirtuins are the longevity genes in mainstream current research about human longevity and immortality, manufactured by almost every cell in the human body.
Sirtuins were discovered as transcription repressors in yeast, however, mammals contain seven enzymes of this family from SIRT1 to SIRT7.
SIR stands for silent information regulator, when SIR2 is written in capitals and italics it stands for genes; when it’s written as sir2 it refers to protein.
Sirtuins remove acetyl tags from histones and some other cytoplasmic proteins, thereby changing the packaging of DNA, which results in turning genes on and off when required[8].
Sirtuins are really ancient genes and possess a highly conserved structure, implying that sequence is maintained throughout the process of natural selection.
These genes control crucial cellular processes, such as DNA repair and reproduction.
Sirtuins function with the help of a molecule NAD (nicotinamide adenine dinucleotide), loss of this molecule as we age, results in a decrease in sirtuin activity.
The SIRT3 enzyme of the sirtuin family is the only enzyme known to have a positive effect on long and healthy lifespans, in humans.
SIRT3 can be found inside mitochondria, main activity performed is the deacetylation of DNA, and helps in maintaining metabolism.
It is shown that people who live relatively longer had higher levels of SIRT3 in their bodies.
A study conducted on mice in 2010 suggests that mice with decreased levels of SIRT3 had increased levels of ROS ( reactive oxygen species) and decreased oxygen levels which posed damage to DNA.
However, other studies about this gene on larger populations weren’t that exciting, stating the effect of SIRT3 is pretty much negligible on larger populations.
Sirtuins are responsible for epigenetic alterations as well, such as histone modification.
Epigenetic modification has a commensurate effect on gene expression of both DNA and histone.
It is believed that manipulation of sirtuins has a positive effect on epigenetic alterations for healthspan modulation.
Target of Rapamycin (TOR)
The Target of Rapamycin (TOR) longevity genes are complex protein structures that manage growth and metabolism.
Just like sirtuins these genes also buckle down the cell, drifting all the energy produced in the cell towards DNA repair and reducing damage caused by senescent cells in stressful conditions.
And perhaps the most crucial function of this gene is to digest old proteins.
However, all these functions and genes are activated when we find ourselves in stressful conditions, which at times are very difficult to overcome.
For example, if you trod over a spider, it dies almost instantly, and no gene is going to help it overcome that “stressful” condition.
Acute trauma kills an organism almost in no time, whether the cell mutates for better survival or not, epigenetic information will suffer a loss anyway.
Yeast Can Help Understand Anti-Aging
You might wonder what that means, it means nothing but the process of understanding the plastic process of aging with the help of yeast.
Yeast and human beings are separated by about a billion years of evolution.
One might ponder upon what’s the significance of this mere microscopic organism.
The answer to that question, quite frankly, is yes, yeasts carry great significance, those minute cells are not so disparate from ourselves.
Their size, their cell structure, and genetic makeup are so complex within themselves, that it makes them a perfect organism to study for various biological processes that sustain life in complex organisms like ourselves.
Yeast is just like humans, either horny or hungry, implying the fact that it is either trying to reproduce or trying to gather nutrition from its surroundings to carry out reproduction.
Fact: Saccharomyces cerevisiae (a strain of yeast) and humans share about 70% of DNA.

As we age, we grow round and less fertile over time despite the fact it takes us decades to do so, but yeast takes about weeks to reach a similar state.
Trying to find the cause of aging in complex organisms like ourselves is a pretty hectic task.
On the other hand yeast cells have similar functionality and similar pathways with a much simpler unicellular structure.
One can easily review the molecular changes associated with aging in an organism like yeast.
The profuse findings from yeast made us discover anti-aging molecules like rapamycin, spermidine, polyphenol resveratrol, etc.
Reverse Aging – Becoming ‘Younger’
There is a major difference between anti-aging and reverse aging, while anti-aging means to halt or slow down the process of aging, reverse aging means to rejuvenate or revive the cells.
Reverse Aging- Yamanaka factors
Back in the year 2006, a scientist named Shinya Yamanaka identified a set of genes within the genome of mice.
Yamanaka factors are: Oct3/4, Sox2, Klf4, c-Myc.
When programmed into the cell (by gene therapy) it could turn back the cell to the immature stem cell phase (stem cells can regrow into any kind of cell).
Shinya called them induced pluripotent cells, this discovery later led him to a Nobel Prize in medicine in the year 2012.
A study conducted on mice in the year 2018, by Dr. David Sinclair and his team used Yamanaka factors (excluding c-Myc, because it is known to cause cancer) on mice to test reverse aging.
They reprogrammed the retina of the 2-year-old mice by gene therapy and it turns out that the retina of those mice start to work like the retina of 2-month-old mice.
This study shows some really promising results, but can we do it on humans?
Is Reverse Aging Possible for Humans?
We do have the ability to reset our epigenomes, but only when we’re at the embryonic stage when there’s an abundant amount of stem cells, but we lose stem cells over time.
Remaining stem cells when we grow older are restricted to the kind of cells it makes.
And even applying gene therapy to each of your trillion cells in the body seems pretty impossible right?
But we do have ways by which we can achieve age reversal, one of which is human genome editing with CRISPR, using which researchers were able to boost the average life span of mice to 215-510 days[9].
Remember when I mentioned the immortal jellyfish in the article, we could maybe understand the ways how to do it by using that jellyfish.
Reverse aging – Hyperbaric Oxygen Therapy (HBOT)
First things first, what is HBOT?
HBOT is a type of treatment used to speed up the healing of carbon monoxide-damaged cells, non-healing wounds, and oxygen-deficit cells.
In hyperbaric treatment, 100% oxygenated air is used at ambient pressure greater than atmospheric pressure at sea level.
By inhaling 100% oxygenated air at higher pressure, 20 times more oxygen goes into cells to repair the damaged tissues.
When patients were treated with HBOT, there was a significant increase in the length of telomeres[10].
And there was a significant decrease in immunosenescence or zombified cells too[11].
There are ways certainly to make us immortal and increase our “healthspan”, and it’s just a matter of time before we actually achieve this feat.
Biologically Immortal Living Beings on Earth
The Immortal Jellyfish

You might have seen a “jellyfish”, blobbing around in deep waters, a particular one we’re gonna be talking about is called the “immortal jellyfish”, they belong to phylum cnidaria.
Its scientific name is Turritopsis dohrnii (T. dohrnii), these jellyfishes return back to their juvenile phase in times of crisis.
It turns back to the polyp state and sinks to the ocean floor, they rapidly undergo this stage and they are basically immortal.
These jellyfish don’t die of age but they are killed by predators (sea slugs or crustaceans).
Hydra – Chunk it to make it

Hydra does not mean that marvel villain that you’re probably thinking of (?).
Hydras are freshwater creatures, belonging to the same phylum as T. dohrnii.
Hydra has the ability to regenerate its body parts, making it biologically immortal.
Basically, it never dies of aging, it just keeps on regenerating.
When will Humans become Immortal?
So far in this article, it seems that mutations in DNA and epigenome are the root of this vicious disease of “aging”, so can’t we just reset the whole DNA in every cell and make good as new (if you’re wondering, it is possible to do so).
But here’s the deal: if we do it our bodies might turn into huge globs of tumors because obviously resetting the DNA will come with a whole lot of other problems, what to direct the cell, what proteins to secrete, it is a huge mess.
Let’s say we find a way to reset the DNA without damaging any information, in clinical trials, but scientists aren’t aware of the long-term effects it may cause.
Would you still do it? If you are a maniac like me, yes for sure (raise a toast! In the name of science).
Will Nanotechnology Make Humans Immortal?
Nanotechnology deals with microscopic bots, as defined by the name measured on the scale of nanometers, roughly about the size of a virus.
Nanobots can be programmed to destroy an undesirable irregularity in the human body.
By the year 2040, it is believed that nanobots will be designed in such a way that they could roam around our bodies and fix the damage in cells.
These bots would be able to move through the bloodstream and treat diseases like cancer, Alzheimer’s, etc.
And perhaps it’ll be able to eliminate all the biological dysfunctionalities and symptoms of aging, paving the way towards life extension and eventually immortality.
If Possible When Will Humans be Immortal?
Human Immortality and longer lifespans come with after-effects of their own, we inhabit a planet with very limited resources and they’re depleting day after day.
All you can do for now is to make some healthy lifestyle changes to keep your mortal body’s systems intact, you know all the stuff your mom tells you to do, consider it (trust me, she knows better).
If any of you stays alive up to 2040-2050, it is possible that immortality is going to be an actual thing with the help of nanotech[12].
Human Immortality can plausibly be a real thing at some time in the future, but not a very certain number for the time.
Mortality accords human life
Data, Star Trek (slightly modified)
YouTube
References
- Max Roser, Esteban Ortiz-Ospina and Hannah Ritchie, ‘Life Expectancy‘, Our World in Data, October 2019, “The United Nations estimate a global average life expectancy of 72.6 years for 2019.’’, https://ourworldindata.org/life-expectancy[↩]
- Ake T. Lu et al. ‘DNA methylation GrimAge strongly predicts lifespan and healthspan | Aging‘, Aging, 21 January 2019, “ DNAm-based estimator of smoking pack-years. Adjusting DNAm GrimAge for chronological age generated a novel measure of epigenetic age acceleration.”, https://www.aging-us.com/article/101684/text[↩]
- Vera Pancaldi, ‘Biological noise to get a sense of direction: an analogy between chemotaxis and stress response‘, Frontiers, 13 march 2014, “Biological noise, here defined as the substantial cell-to-cell variation that is observed in populations of genetically identical cells.”, https://www.frontiersin.org/articles/10.3389/fgene.2014.00052/full[↩]
- Yixin Yao and Wei Dai, ‘Genomic Instability and Cancer‘, PMC, 25 February 2014, “The maintenance of genomic stability are essential for cellular integrity to prevent errors from DNA replication.”, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4274643/[↩]
- Simona Negrini, Vassilis G Gorgoulis, and Thanos D Halazonetis, ‘Genomic instability-an evolving hallmark of cancer‘, PubMed, n.d. “ Genomic instability is a characteristic of most cancers. In hereditary cancers, genomic instability results from mutations in DNA repair genes and drives cancer development.”, https://pubmed.ncbi.nlm.nih.gov/20177397/[↩]
- Masood A.Shammas, ‘Telomeres, lifestyle, cancer and aging‘, PMC, 8 June 2012, “Telomere length shortens with age. Progressive shortening of telomeres leads to senescence, apoptosis, or oncogenic transformation of somatic cells, affecting the health and lifespan of an individual., https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3370421/”[↩]
- MedlinePlus, ‘What is epigenetics‘, MedlinePlus genetics, n.d, “When methyl groups are present on a gene, that gene is turned off or silenced, and no protein is produced from that gene, the chemical groups influence how tightly the DNA is wrapped around histones, which affects whether a gene can be turned on or off.”, https://medlineplus.gov/genetics/understanding/howgeneswork/epigenome/[↩][↩]
- Wioleta Grabowska, Ewa Sikora and Anna Bielak- Zmijewska, ‘Sirtuins, a promising target in slowing down the aging process‘, PMC, 3 March 2017, “Sirtuins were originally discovered as transcription repressors in yeast, however, nowadays they are known to occur in bacteria and eukaryotes (including mammals). In humans, the family consists of seven members (SIRT1-7) that possess either mono-ADP ribosyltransferase or deacetylase activity.”, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5514220/[↩]
- David R. Liu et al., ‘In vivo base editing rescues Hutchinson–Gilford progeria syndrome in mice’, Nature, 6 January 2021, “A single injection of ABE-expressing AAV9 at postnatal day 14 improved vitality and greatly extended the median lifespan of the mice from 215 to 510 days.”, https://www.nature.com/articles/s41586-020-03086-7[↩]
- Yafit Hachmo et al. ‘Hyperbaric oxygen therapy increases telomere length and decreases immunosenescence in isolated blood cell‘, Aging, 18 November 2020, “Telomeres length of T helper, T cytotoxic, natural killer and B cells increased significantly by over 20% following HBOT.”, https://www.aging-us.com/article/202188/text[↩]
- Yafit Hachmo et al. ‘Hyperbaric oxygen therapy increases telomere length and decreases immunosenescence in isolated blood cell’, Aging, 18 November 2020, “There was a significant decrease in the number of senescent T helpers.”, https://www.aging-us.com/article/202188/text[↩]
- P.S Aithal, and Shubhrajyotsna Aithal., ‘Nanotechnology Based Innovations and Human Life Comfortability – Are We Marching Towards Immortality?’, SSRN, 30 November 2018, “It is also predicted that nanorobots could slow or even reverse the aging process, and life expectancy of human beings could increase significantly.”, https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3289262[↩]





